Powder metallurgy refers to a wide range of methodologies through which parts are fabricated starting from metal powders.
In general, powder metallurgy allows to manufacture objects with a high level of precision, in most cases obtaining near net shape parts, reducing or totally avoiding the need for further machining thereby lowering the amount of wasted material, lead time and, in general, total costs.
Several technologies have been used for decades to transform powder into shaped components, such as die pressing, isostatic pressing, press and sintering, Metal Injection Molding (MIM) and also, more recently, Powder Bed Fusion (PBF) and Direct Energy Deposition (DED) additive manufacturing.
Vacuum furnaces are an essential part of several of these powder metallurgy technologies at various steps; for example, MIM, Binder Jetting (BJT) and metal FDM all rely on vacuum furnaces for the removal of the binder and sintering of the parts after the injection (in the case of MIM) or the printing (in case of BJT and metal FDM) process. Powder Bed Fusion technologies instead, such as Selective Laser Melting (SLM) and Electron Beam Melting (EBM), employ vacuum furnaces to heat treat the printed parts: stress-relieving, annealing, solution treatment and aging of PBF parts are carried out in vacuum furnaces to provide the desired parts properties minimizing the risk of oxidation and contamination during the treatment.
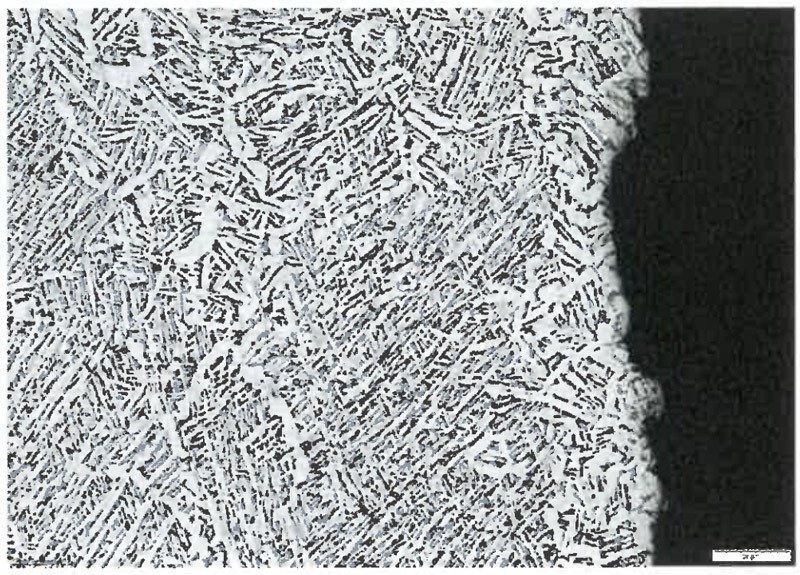
Figure 1: Metallographic image of a Ti-6Al-4V part heat treated in a vacuum furnace showing the presence of a mixed α-β structure. Heat treatment is critical to obtain the desired microstructure on AM parts.
A critical element for all of the powder metallurgy manufacturing processes is, you’ve guessed it, the powder.
A basic knowledge of the main metal powder properties and production methods is essential to understand and predict how the powder will behave at each step of the process, whether it is the pressing, the mixing with a binder, the injection or the sintering and to select the correct process parameters for each of those.
For that reason, in this article we’re going to look into the powder production methods and the properties used to characterized metal powders.
Figure 2: TAV H3 All-Metal designed for heat treatment of AM parts
Powder Production
Different production techniques are employed today to produce virtually all metal powders.
Methods for metal powder production can be divided into mechanical (such as milling, mechanical alloying, water and gas atomization) and chemical methods (such as electrolysis and reduction of oxides), with the first ones generally allowing higher production rate and better volume to cost ratio and the latter delivering higher purity powder.
Let’s go more in depth of each powder production method.
Milling/Comminution
This method relies on progressive impact from one or several tools, such as hammers, rod mills or balls, to form the metal powder.
Comminution is the most widespread method for the production of hard metal powders and ceramic oxide powders (e.g. Al2O3 and ZrO2). Since the powder particle are produced through random fractures induced by the tool, the particles size distribution obtained via mechanical comminution is usually wide and the particles dimensions can range between 1 µm to 1000 µm. Particles produced by mechanical techniques are usually irregular and angular shaped. Ideally, materials to be mechanically pulverized need to be brittle; ductile materials in fact will flatten and deform under the mechanical stress without fracturing. For that reason, milling is usually applied to ductile materials after forming a transient brittle phase: the metal is oxidized to form brittle oxides, then milled and finally heated in vacuum or in a hydrogen atmosphere to reduce the oxides.
Milling can also be applied to metal powders to modify the particles size, change the particles shape or to mix two or more materials through solid-state alloying (mechanical alloying) or solid-state blending (incomplete alloying).
Atomization
Atomization is the most common production method for metal and prealloyed powders.
Powder atomization relies on the reduction of a liquid into small droplets that are then condensed, forming powder particles: the metal is melted in vacuum, conveyed into a nozzle together with high pressure gas or water jet and then condensed into a collection chamber. The dimensions of atomized powder particles can range between 10 µm up to 1mm depending on the type and pressure of the media used to form the liquid metal jet. Gas atomized powder typically exhibits a spherical shape, high surface purity and high packing density. Water atomized powder, in comparison, usually has slightly irregular shaped particles, even though the use of a very high water pressure results in the finest particles.
Chemical-Thermochemical Reactions
Several chemical and thermochemical reaction are used as means to form metal powder, including:
- Oxide reduction
- Precipitation from solution
- Thermal decomposition
Oxide reduction
This process utilizes a reducing agent (such as carbon or hydrogen) to reduce oxide powder into metal powder. The powder particle size is dependent on the reaction temperature and on the subsequent attrition or milling, which is usually mandatory to obtain an adequately fine particle size.
Precipitation from solution
Electrochemical precipitation from a solution is applied to the formation of elemental powders.
An electric current drives a plating reaction; the electrodeposit is then intentionally disrupted to form a porous product that is a precursor to the powder. The sponge deposit is collected cleaned, dried and milled into powder. Electrochemical precipitation is not applicable to alloys since each element has a different characteristic deposition window.
Powder Characterization
Characterizing a metal powder means measuring its physical and chemical properties to determine how the powder is going to behave.
In the following, we’re going to list the main powder properties used for metal powders characterization.
Particle size distribution
The particle size distribution of a powder indicates the relative amount of particles present according to their size. The particle size may be defined according to their surface area, projected area, volume or mass. Usually particles are assumed to be spherical. One of the most common means of measuring particle size distribution is light scattering using laser, where the projection of the light diffracted on particles suspended in a liquid is measured. It is applicable to particles in the 0.1-1000 µm range, which basically includes all of the powder used in the MIM and AM industries.
Density
When talking about the density of a powder it is important to specify what type of density we are referring to. We can distinguish between:
- Bulk density (or apparent density): it’s the ratio of the mass of an untapped powder sample (i.e. loose powder without any compaction) and its volume, including the contribution of interparticulate voids
- Tapped density: it’s the density attained after mechanically tapping the powder (i.e. compacting the powder until the apparent volume of the sample becomes almost constant).
Powder density is strictly related to other properties such as shape and roughness of the powder particles; finer particles, for example, will lead to a higher bulk density value.
Powder Flowability
Powder flowability is a measure of the tendency of powder particles to move among each other or among an external surface. Flowability is a critical parameter for powder metallurgy, since powders with different flowability will fill dies, hoppers or spread on the build platform of an SLM system differently.
The main variables affecting the flowability of a powder are: interparticle friction (which in turn depends on particles shape and roughness), particle size, material, specific weight and external environmental factors (such as temperature, humidity or presence binders/lubricants).
Alloying Method
Most of the metal powders used in the powder metallurgy industry are not pure elements but metal alloy. Alloying methods could be divided into:
- Elemental method: blending elemental powders with calculated ratios to form the final stoichiometry of the alloy.
- Prealloyed method: the powder is produced starting from a material that already has the exact stoichiometry of the alloy of interest.
- Master alloy method: the powder is produced starting from prealloyed material which is enriched in certain alloying elements, and then mixed with elemental powders to reach the exact stoichiometry of the alloy of interest.
Master alloyed steel powders may have a higher native carbon content: for that reason appropriate measures have to be taken to ensure carbon control when debinding an sintering these sort of powders.